MAGNETOENCEPHALOGRAPHY (MEG) CAPTURE
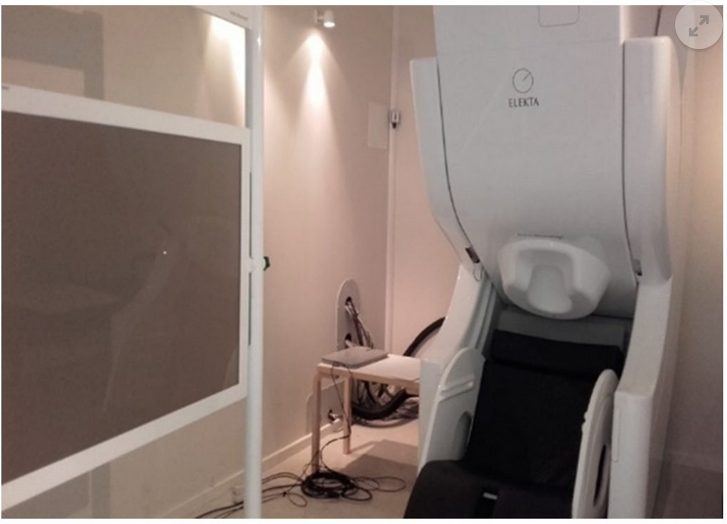
Magnetoencephalography (MEG) is a technique that records brain function activity, by capturing the magnetic fields produced during neuronal synapse which are projected outwards. MEG is, therefore, a non-invasive technique, which does not require any kind of preparation of the patient (e.g., fasting, administering medicines, or injecting radioactive markers, etc.) and does not subject them to any kind of radiation or electromagnetic field. The magnetoencephalogram is helmet-shaped, with sensors all over the cranial convex. To prevent noise coming from unwanted signals, the equipment is inside a room that is shielded from the exterior.
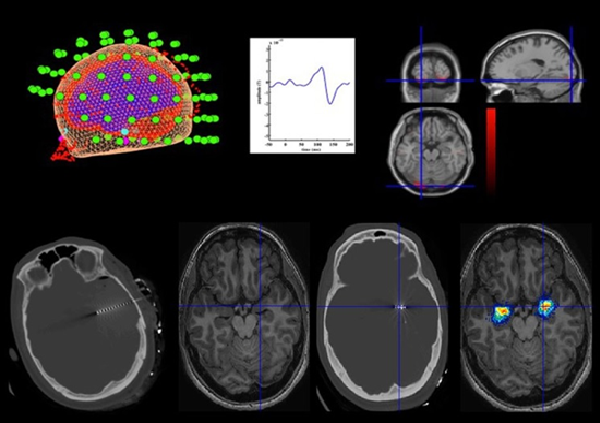
MEG measures the magnetic fields produced by neuronal electrical activity, with a high temporal resolution and good spatial resolution, enabling its sources to be identifies and the relations between cerebral structures and their functions to be researched.
The Elekta-Neuromag Magnetoencephalography equipment is located at the Biomedical Technology Centre (BTC). It has 306 sensors (a combination of 204 gradiometers and 102 magnetometers) and has one of the most modern designs for this kind of technology. It also has a blink control for patients with Epilepsy.
There are three work stations linked to the system, one for acquisition and the other two for analysis, that have software provided by the company as well as the Cogent, Eprime, Presentation and Stim programs.
Furthermore, using a 32 channel electroencephalogram coupled to the MEG equipment, it is possible to take simultaneous electroencephalography (EEG)/MEG records. There is also a 64 channel stand-alone EEG machine made by Neuroscan.
A helium recycler enables consumption of the coolant, which is used to get maximum sensitivity and precision in the magnetic field sensors, to be minimised.
Needs requested and applications
The development of MEG in the university and health field involves a boost to a whole series of lines of research and progress in diagnosing diseases such as Alzheimers, epilepsy, strokes, Parkinsons, and head trauma, etc., which are diseases that come with a high economic and social cost. The inclusion of MEG into the clinical/diagnostic process enables progress in preventive medicine and health promotion.
Sector or area of application
Research and healthcare
Differential skills
The Biomedical Technology Centre at the Universidad Politécnica de Madrid has the only magnetoencephalography (MEG) equipment in the whole Community of Madrid and the only MEG equipment in the whole of Spain that offers its services to the Scientific and Social Community, covering the field of Research as well as the Clinical area.
The Magnetoencephalography capture and its analysis is carried out by the Clinical Neuroscience Laboratory at the Biomedical Technology Centre, which has a multi-disciplinary team (Engineers, Neurologists, Neuropsychologists, Nerophysiologists and Psychologists) with extensive experience of over 13 years and more than 3,000 cases resolved in various neurological diseases (Epilepsy, Strokes, ADHD, Drugs, Cognitive Impairment and Alzheimers).
The Biomedical Technology Centre has health clearance to operate as a C.3 (Health Service Integrated into a non-Health Organisation with a U.1 General Medicine unit, U.17 Neurology, and U.900 Other healthcare units (Clinical Psychology)), granted by the Community of Madrid Health Department on 25/11/2019.
Previous references for provision of services
The MEG services offered by the BTC are currently in demand from the following users:
In-house (UPM) Users
At the Biomedical Technology Centre the following laboratories require the use of the MEG for their daily research work:
- Clinical Neuroscience Laboratory
- Experimental Neurology Laboratory
- Active Ageing Laboratory
Non-UPM Users
As a result of research collaborations (joint or coordinated projects and research agreements), doctors and researchers from the following institutions regularly access the MEG:
- Public and Private hospitals: Hospital Clínico San Carlos, Hospital Ruber, Hospital La Princesa, Hospital 12 de Octubre
- Universities: Complutense de Madrid, Balearic Islands and Castilla la Mancha
- Companies: Jerome Lejeune Foundation, Sincrolab SL, Bitsphi SL, Brain Investigations SL, Neurología Aplicada SL
Where it is
The magnetoencephalography system is located at the Biomedical Technology Centre (Montegancedo Campus) in the Clinical Neuroscience Laboratory on floor -2 MEG (35 A S2.10).
Request for service
For proper use of the MEG equipment, the suitable compatibility of its use as a research tool offering services to the clinical and research community, and its contribution to the sustainability and improvement of its parts and infrastructure, it has been considered necessary to set out an access and use procedure which is different depending on the purpose of the study: Researcher or Clinician.
Researchers from the BTC or other public and private entities may have access to the equipment when they request it via an e-mail to the SCIENTIFIC RESPONSIBLE FOR THE SERVICE Dr. Bryan Strange: .
Every application must be accompanied by the following documentation:
– A research memorandum consisting of the following sections
Summary (300 words)
Introduction current state of the matter (one page)
Aims and assumptions (one page)
Experiment design and study method (one page), including
Experiment design description, including the dependent and independent variables
Description of the cognitive work design to be carried out during the MEG capture
Description of the resting state and when it should be carried out
Description of the subject sample
Timetable (one page) including suggested start and end dates.
Resources to do the project (one page)
– Approval from an official ethics committee (hospitals, clinical foundations and pharmaceutical laboratories). At any event, all projects will be assessed by the UPM ethics committee.
– Documentary proof of having applied for, or obtained, funds to carry out the research. The viability between objectives and available funds will be assessed by the SCIENTIFIC RESPONSIBLE FOR THE SERVICE Dr. Bryan Strange: ">. The feasibility of the applications and their temporal organisation will be assessed by a committee chaired by the SCIENTIFIC RESPONSIBLE FOR THE SERVICE.